Current Projects
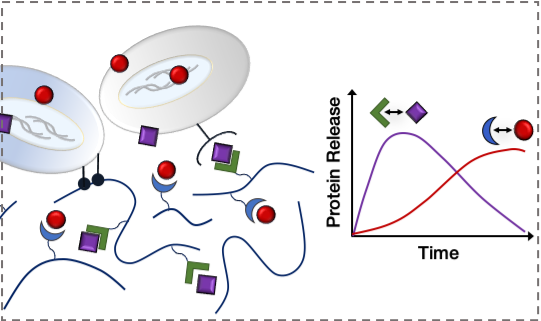
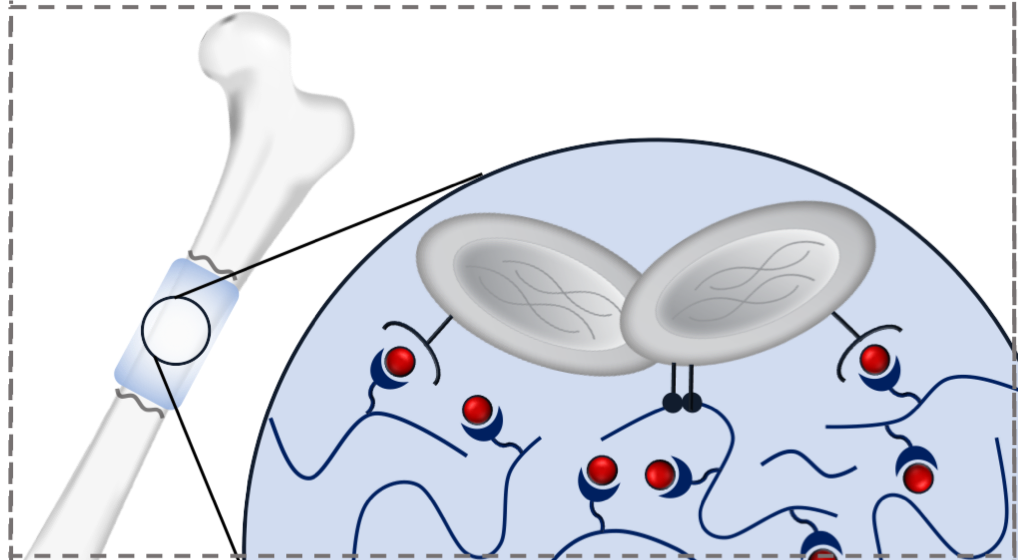
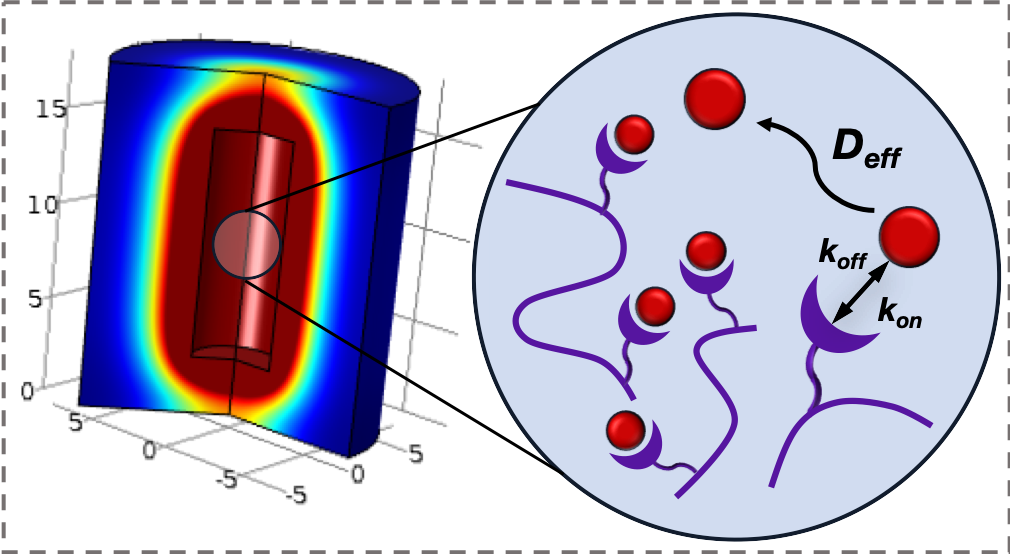
Affinity-based Biomaterials for Controlled Protein Delivery
Current strategies for controlling protein delivery from biomaterials rely on natural protein-material interactions with limited tunability or the expression of challenging fusion proteins. We are using a combination of computational protein modeling and display platforms to generate specific affinity interactions with proteins of interest, such that their local presentation and effect on different stages of tissue healing may be tuned with precision and flexibility. This approach will enable the controlled delivery of multiple therapeutics from a single biomaterial delivery vehicle.
Cell-Instructive Biomaterials for Bone Repair
Stem cell transplantation can stimulate tissue repair through the secretion of pro-regenerative paracrine factors. Harnessing the regenerative and immunomodulatory potential of stem cells through their secretome represents a recent paradigm shift in the field of tissue engineering. We are creating biomaterial delivery vehicles that facilitate both paracrine- and cell-based tissue repair strategies by (1) presenting biochemical cues that promote cell viability and encourage paracine factor secretion of stem cells, and (2) selectively sequestering paracrine factors in the injury micro-environment using affinity-based biomaterials. We aim to prolong local presentation of secreted proteins beyond the initial period of transplanted cell survival and create a pro-regenerative environment within injury sites.